- 글번호
- 949871
Prof. Yangmi Kim’s Team Identifies a Principle of Antibacterial Peptides from Insects for Sepsis Treatment
- Writer
- 관리자
- View
- 2620
- Date
- 2022.03.04
- 수정일
- 2022.03.04


A research team of Prof. Yangmi Kim (Department of Systems Biotechnology) at Konkuk Institute of Science and Technology (KIT) increased the possibility of developing treatments for sepsis by identifying the principles of antibacterial peptides from insects.
The team identified the principle of treating sepsis by controlling TLR 4 by combining antibacterial peptides from insects with endotoxin (LPS), an extracellular component of gram-negative bacteria, through structure-based interaction. The research was published online on March 1st in the international journal, ‘Proceedings of the National Academy of Sciences of the United States of America (PNAS)’.
When our body is infected with gram-negative bacteria, TLR4, which plays an essential role in the innate immune system, forms a complex with MD-2 protein and perceives the molecular pattern of LPS, causing an immune response. During the process, the excessive immune response caused by LPS results in cytokine storms and fatal sepsis. Unlike higher animals such as humans that defend their bodies by operating acquired immune systems, insects defend against the invasion of pathogens only with the innate immune system while secreting innate immune factors including antibacterial peptides that have sterilization capabilities.
Prof. Kim’s research team has found out that papiliocin, a cecropin-based antibacterial peptide from insects, removes LPS with a bonding force 10 times stronger than polymyxin, which is known for the last antibiotic of gram-negative bacteria treatment. With a similar bonding pattern interfering bonds of LPS and TLR4, the team has also identified a core principle that fundamentally treats sepsis by controlling excessive TLR4 immune response. Based on the similarity between the molecular structure of antibacterial peptides, which are innate immune factors of insects, and the LPS molecular pattern of gram-negative bacteria, Prof. Kim suggested that the TLR4-mediated immune response regulation in humans that perceives LPS and the innate immune response including insects’ toll receptors will be structurally and functionally similar.
While researching the three-dimensional structure of protein and anti-bacterial peptides, Prof. Kim has developed various new drugs and commercialized antibiotics and commercialized them as antibiotics and cosmetic materials. Based on the research results, Prof. Kim confirmed the effectiveness of sepsis treatment with 12 new peptide residues and is developing it as a treatment for gram-negative bacteria. The DD-S052 peptide sepsis treatment, recently developed by Professor Kim based on the bonding structure with LPS through joint research with Dandi Bioscience, is scheduled to enter phase 1 clinical trials this year after the preclinical test is completed.
Prof. Kim said, “Due to the overuse of antibiotics in recent years, we have faced the emergence of super-bacteria and rapidly increasing mortality rates of sepsis from bacterial infection,” and, “This research is expected to have a great ripple effect by securing original technology for the development of resistant bacteria treatments.”
The research was conducted with the support of mid-sized research projects by the Ministry of Science and ICT.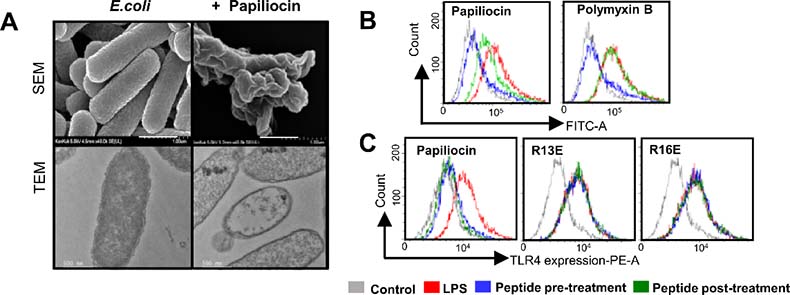
Image 1. (A) Unlike the existing antibiotics, cecropin-based antibacterial peptides from insects show a great capability to fight against innate gram-negative bacteria by destructing its cell membranes. (B) Unlike polymyxin, papiliocin not only interrupts LPS combining with cell membrane receptors but controls the excessive immune response of TLR4 by better removing the LPS that have already been combined. (C) When the Arg13 and Arg16 residues, which play a significant role in combing TLR4 and peptides, are replaced by Glu or Ala, they lose the activity of controlling the TLR4 immune response.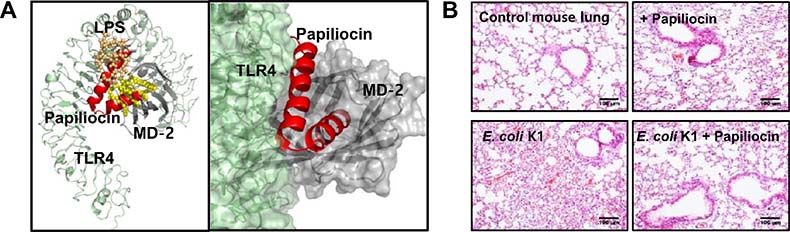
Image 2. (A) Comparing the bonding structure of TLR4/MD-2 and LPS (3FXI) and the bonding structure of papiliocin, it is shown that LPS and peptides have a similar bonding pattern. Papiliocin with 37 residues is composed of two spiral structures meanwhile hydrophobic spiral structures combine with a hydrophobic pocket of MD-2 and amphiphilic spiral structures bind to the interface between TLR4 and MD-2. Thus, it controls the excessive immune response of TLR4 by interrupting the bonding of LPS and TLR4/MD-2 which have similar bonding patterns. (B) It is confirmed that papiliocin in sepsis animal model experiments inhibits cytokine storm resulting from the excessive immune response of TLR4 and treats organ damages such as lungs or kidneys.